NeuraMedica Inc. Receives FDA 510(k) Clearance for DuraFuse Dural Clips for Fast & Secure Dural Closure
Tuesday, August 30th, 2022
NeuraMedica Inc., has received 510(k) FDA clearance for DuraFuse™ Dural Clips
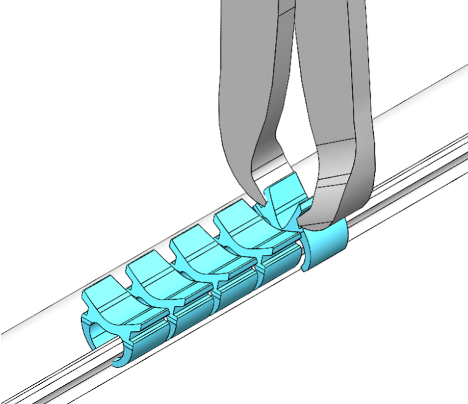
Oregon City, Oregon, August 30, 2022 – NeuraMedica Inc., a medical device company developing innovative products for neurosurgery, has received 510(k) clearance for DuraFuse™ Dural Clips from the U.S. Food and Drug Administration (FDA).
“The idea for a dural clip came to me when I was a neurosurgery resident at OHSU [Oregon Health & Science University]. I realized how time-consuming suturing the dura can be and I knew there had to be a better way, especially in cases with minimal access.” said Neil Roundy MD, a neurosurgeon at Oregon Neurosurgery in Springfield, Oregon and Co-Founder and Chief Medical Officer (CMO) of NeuraMedica.
DuraFuse Dural Clips allow neurosurgeons and orthopedic spine surgeons to close incidental and planned spinal durotomies in a fraction of the time it would take to suture the dura. The dura mater, which covers and protects the brain and spinal cord, must be repaired as quickly as possible when opened to prevent cerebrospinal fluid (CSF) leakage. Traditional dural closure with suture can be time-consuming and difficult. Metallic clips are permanent and can cause imaging artifacts. DuraFuse Dural Clips are a non-penetrating, bioabsorbable, imaging-compatible device designed to create a fast and secure dural closure in both open and tubular retractor procedures of the spine, saving surgeons and hospitals significant time and money.
“DuraFuse Dural Clips represent a significant advance for surgeons who need to close spinal dura quickly. We are thrilled to receive FDA 510(k) clearance and bring DuraFuse to market,” said Rachel Dreilinger, Co-Founder and Chief Executive Officer (CEO) of NeuraMedica.
NeuraMedica is looking forward to our full commercial launch in late 2022. The company plans to attend the National Association of Spine Specialists (NASS) Annual Meeting in Chicago, Illinois in October 2022.
“We are very excited with the initial response we are seeing for DuraFuse. This product represents a simple, safe, and effective way for surgeons to close the dura,” said Chris Eddy, VP of Sales at NeuraMedica.
To learn more about DuraFuse Dural Clips, visit NeuraMedica.com/durafuse-spinal-dural-clip.
About NeuraMedica Inc.: NeuraMedica is a medical device company specializing in innovative products for neurosurgery. NeuraMedica is a woman-owned and Native-owned (Diné) company founded in 2014 by biomedical engineer Rachel Dreilinger and neurosurgeon Neil Roundy MD to develop a straightforward solution to dural repair that benefits surgeons, hospitals, and patients. Commercialization of this product was made possible by funding from the National Science Foundation (NSF), the National Institutes of Health - National Institute of Neurological Disorders and Stroke (NIH NINDS), OHSU Oregon Clinical and Translational Research Institute (OHSU OCTRI), Oregon Nanoscience and Microtechnologies Institute (ONAMI), Business Oregon, and Alignment Ventures. To learn more about NeuraMedica, visit NeuraMedica.com.



 March 5 – April 9
March 5 – April 9 Register now.
Register now.